Language selection
- Français fr

Statement on COVID-19 and International Travel
Last content update: December 21, 2022
An Advisory Committee Statement (ACS) Committee to Advise on Tropical Medicine and Travel (CATMAT)
Table of contents
Key points/messages, assessing individual travel associated sars-cov-2 risk, host vulnerability assessment, other considerations, recommendations, acknowledgements, conflicts of interest, appendix: types of activities and the relative likelihood of sars-cov-2 exposure.
The Committee to Advise on Tropical Medicine and Travel (CATMAT) provides the Public Health Agency of Canada (PHAC) with ongoing and timely medical, scientific, and public health advice relating to tropical infectious disease and health risks associated with international travel. PHAC acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and medical practices at the time of writing, and is disseminating this document for information purposes to the medical community caring for travellers. Persons administering or using drugs, vaccines, or other products should also be aware of the contents of the product monograph(s) or other similarly approved standards or instructions for use. Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) or other similarly approved standards or instructions for use by the licensed manufacturer(s). Manufacturers have sought approval and provided evidence as to the safety and efficacy of their products only when used in accordance with the product monographs or other similarly approved standards or instructions for use.
- Travel advisors and their clients should verify any requirements (COVID-19 related or otherwise) in place at their destination(s) and for their return to Canada .
- CATMAT strongly recommends eligible travellers be adequately vaccinated with a Health Canada authorized COVID-19 vaccine before travel.
- The approach to vaccination, including the number and spacing of doses, and approaches for special populations, should be as recommended by the National Advisory Committee on Immunization (NACI) . Current NACI recommendations on the use of COVID-19 vaccines can be found in the COVID-19 vaccine chapter of the Canadian Immunization Guide.
- Generally, CATMAT recommends against receiving the COVID-19 vaccine while in other countries.
- Infants who are not eligible for COVID-19 vaccines in Canada should not be vaccinated when travelling, even if the product is the same as those authorized for adolescent/adult use in Canada, or is authorized for use in children in other countries.
- In the absence of vaccination, or in the case that individuals have a diminished immune response to the vaccine, or are otherwise at an elevated risk for severe outcomes from infection, special attention should be paid to the use of individual public health measures for protection during travel.
- CATMAT suggests that, at the individual level, the main consideration when advising patients on travel in a COVID-19 environment should be the risk of severe COVID-19 associated outcomes. Accordingly, host vulnerability is the primary focus of the present guidance (see Table 2 ).
- Additional considerations include, the potential for transmission to others (particularly those who are higher risk for severe COVID-19), access to adequate medical services while abroad, potential for local healthcare facilities to be overwhelmed, and potential disruptions to travel as the COVID-19 situation evolves.
- The results of the host vulnerability assessment (ranging from low to very high) are explicitly linked to recommendations (see Table 2 ).
- For individuals considered at very high likelihood of developing severe COVID-19 if infected ( Table 2 ), travelling with standby treatment of antiviral medication (e.g., nirmatrelvir/ritonavir) may be considered, particularly where access to good quality medical care and treatment is anticipated to be limited.
- CATMAT acknowledges that availability for this purpose may be limited, as eligibility criteria across most jurisdictions in Canada currently require a positive test result and mild/moderate symptoms.
- Pre-exposure prophylaxis for COVID-19 may be indicated in certain high-risk populations irrespective of travel plans Footnote 1 . Travellers who are immune suppressed should discuss the use of monoclonal antibodies with their health care provider. If monoclonal antibodies are indicated and the traveller has access to treatment, they should initiate treatment prior to travel.
- Monoclonal antibodies have a continuously evolving role, and travellers should consult an expert to determine the most appropriate use of these agents for their individual situation and itinerary.
- Serologic testing for antibodies pre- or post-travel, as a surrogate for protection, is not recommended. There is no adequately validated correlation between serologic results from a given test and risk of severe outcomes.
This statement provides guidance for health care providers advising patients on health-related aspects of travel in a COVID-19-affected world. Policy and regulatory aspects of the COVID-19 response, such as requirements around vaccination and quarantine, are outside of the purview of this statement. For more information in this regard, travel advisors and their clients should, among other things, regularly verify any travel requirements in place at their destination(s) and for their return to Canada .
This statement was developed by members of a CATMAT working group (WG), none of whom declared a relevant conflict of interest. This guideline was developed based on expert opinion informed by a narrative review of relevant evidence. The final statement and recommendations were approved by CATMAT.
Clinical and epidemiologic aspects of coronavirus disease 2019 (COVID-19) are well described elsewhere. The WHO COVID-19 Weekly Epidemiological Update , for example, provides up-to-date information on the global epidemiological situation as well as emerging knowledge related to SARS-CoV-2 variants of concern (VOCs) and variants of interest (VOIs).
There are many factors that might affect the decision to travel during the ongoing SARS-CoV-2 pandemic. Some are structural, including travel and border regulations and requirements. This guideline does not consider these aspects, nor does it consider indirect but very important impacts of individual travel on others, for example the situation where a healthy traveller with mild illness may transmit SARS-CoV-2 to someone who is more vulnerable, or the potential for travel-associated spread of VOCs. Rather, the focus of this guidance is on :
- assessment of the traveller's (i.e. the host) vulnerability to severe COVID-19 related outcomes ( Table 1 ); and,
- risk-management recommendations based on the host vulnerability assessment.
A key assumption within this guideline is that, in many circumstances and for a variety of reasons (including lack of travel health insurance coverage for COVID-19), travellers afflicted with COVID-19 will have reduced ability to access timely and/or sufficient health care support during their trip.
This guidance does not explicitly consider the reported level of SARS-CoV-2 transmission at a destination in our assessment. While there are important transmission heterogeneities between destinations, it is becoming increasingly difficult to accurately assess these due to differences in testing, surveillance, and reporting architectures. However, health care advisors should consult with Government of Canada Travel Advice and Advisories for destination-specific travel information and advice.
Finally, this guidance does not consider values and preferences of the traveller. Given that travellers will have divergent COVID-19 risk tolerances, it follows that their acceptance of travel recommendations will vary. It is in this context that travel-related decisions will be made, and where health care providers can help travellers make informed choices.
The host vulnerability assessment estimates the qualitative likelihood that, if infected, a traveller will suffer significant acute harms, i.e. severe disease. The assessment framework was developed based on expert opinion, informed by a narrative review of relevant evidence. Age serves as the baseline for the host vulnerability assessment, given the well-established evidence of an increasing relative risk of severe COVID-19 outcomes with increasing age. Underlying medical conditions associated with an increased risk of severe outcomes from COVID-19 are based on evidence from systematic reviews or meta-analyses. Generally, CATMAT does not differentiate between conditions with respect to the resulting impact on the host vulnerability assessment.
Vaccination status
The most important modifiable risk-management intervention for protection against severe COVID-19 is vaccination.
For most persons who have been adequately vaccinated (meaning, in accordance with NACI recommendations ) and are expected to mount an appropriate response based on their known immune status, CATMAT suggests reducing the host vulnerability assessment by one level (shift to the left) from the baseline age-based estimate (see Table 1 ).
This approach means, for example, a person otherwise considered at high likelihood of significant SARS-CoV-2 infection-related harms based on the host vulnerability assessment, would be considered at moderate likelihood, if appropriately vaccinated as per NACI guidance. Medical advisors should stay up-to-date with the latest guidance from NACI as vaccine recommendations may evolve over time.
COVID-19 related travel requirements and border measures
Recommendations applied in this guidance may differ from those applied to policy around travel requirements or border measures. Travellers should be advised to monitor both the requirements at their destination(s) , and in Canada , including relevant provincial/territorial and local legislation , regulations, and policies, and that these could change during their travel period.
Host-based vulnerability
Age has the most profound population-level impact on the likelihood of severe COVID-19 and serves as a baseline for the host vulnerability assessment Footnote 2 Footnote 3 Footnote 4 (see Table 1 ). Importantly, age is confounded with other risk factors, particularly co-morbidities, such that the independent impact of age is reduced in adjusted analyses Footnote 2 Footnote 3 Footnote 4 . Nonetheless, public health authorities use age as a fundamental baseline for assessing host vulnerability to, and consequent recommendations for the prevention of, COVID-19.
In general, if a traveller has been vaccinated in accordance with NACI recommendations , then the host vulnerability assessment can be reduced (shifted to the left) by one level, from the baseline age-based assessment.
Children under 6 months of age, for whom vaccines are not authorized
Very young, healthy children, for whom COVID-19 vaccines are not (at the time of writing) authorized are considered to be at very low risk for severe COVID-19 associated outcomes (barring any underlying medical conditions), even in the absence of vaccination Footnote 5 Footnote 6 Footnote 7 Footnote 8 .
Host vulnerability factors
The impact of other risk factors on the most serious COVID-19 outcomes is an area of ongoing investigation Footnote 9 Footnote 10 . A summary list of underlying medical conditions associated with more severe COVID-19 disease has been developed by the Public Health Agency of Canada ( Box 1 ). In general, the risk of more severe disease increases with the number of medical conditions.
Box 1. Underlying medical conditions associated with increased risk for severe outcomes from COVID-19
Medical conditions (extracted from COVID-19 signs, symptoms, and severity of disease: A clinician guide from PHAC) Footnote a Footnote b
- Cerebrovascular disease
- Chronic kidney disease
- Chronic liver diseases (limited to: cirrhosis, non-alcoholic fatty liver disease, alcoholic liver disease, and autoimmune hepatitis)
- Chronic lung diseases (limited to: bronchiectasis, chronic obstructive pulmonary disease, interstitial lung disease, pulmonary hypertension, pulmonary embolism)
- Cystic fibrosis
- Diabetes mellitus (type 1 or type 2)
- Disabilities (e.g. Down syndrome, learning, intellectual, or developmental disabilities; ADHD; cerebral palsy; congenital disabilities; spinal cord injuries)
- Heart conditions (e.g. heart failure, coronary artery disease, cardiomyopathies, etc.)
- HIV infection
- Mental health disorders (limited to: mood disorders, including depression; schizophrenia spectrum disorders)
- Pregnancy and recent pregnancy
- Primary immunodeficiency diseases
- Smoking, current or former
- Solid organ or blood stem cell transplant
- Tuberculosis
- Use of corticosteroids or other immunosuppressive medication
For the most current list of conditions, health care advisors should refer to: COVID-19 signs, symptoms, and severity of disease: A clinician guide
Return to footnote a referrer
To aid in assessing immune suppression, a list of conditions under which individuals would be considered moderately or severely immunocompromised can be found in the COVID-19 vaccine chapter of the Canadian Immunization Guide .
Return to footnote b referrer
Generally, CATMAT recommends increasing the host vulnerability assessment (shifting to the right) by at least one level if the traveller has any underlying medical condition in Box 1 . The effect of vaccination (leftwards shift) and medical conditions (rightwards shift) are additive. It is recognized that this is a conservative approach, as some or all of the effect associated with the other identified individual risk factors is likely already factored into the age-based groupings.
In the situation that the health advisor considers the traveller to be especially vulnerable to COVID-19 (independent of age), such as due to the presence of two or more factors or particularly severe medical conditions, consider shifting the host vulnerability assessment to the right by two levels (indicating a larger increase in the risk of severe SARS-CoV-2 related harms). Box 2 provides examples of host vulnerability assessments.
Due to the relative paucity of evidence on the efficacy and effectiveness of vaccination in preventing severe outcomes from COVID-19 among populations with various known types of immune suppression, CATMAT recommends a conservative approach in applying the host vulnerability assessment to these individuals. Many or all will derive some protection from vaccination and it is strongly advised that NACI guidance on the use of COVID-19 vaccines in this population continue to be followed and closely monitored. Evidence continues to evolve on how to optimize protection in this diverse population Footnote 11 .
If the traveller has any of the immune suppressive conditions under which individuals would be considered moderately to severely immunocompromised , found in the Canadian Immunization Guide, CATMAT maintains the recommendation to increase the host vulnerability assessment (shift to the right) by at least one level. For immune suppressive conditions, or for immune suppressing medications, the clinical implications will vary significantly among travellers and should be subject to individual assessments and clinical discretion.
Immune suppressed travellers may be at risk both due to their underlying disease or due to the immunosuppressive medications they are taking and because their protection from vaccine may be reduced. Accordingly, providers may consider during an individual assessment whether the host vulnerability assessment should be increased (shifted to the right) by more than one level. In some cases and based on consultation with a medical expert, it may be appropriate to treat these individuals in the host vulnerability assessment as not being adequately protected by vaccine, regardless of their actual vaccination status.
Box 2. Examples of host vulnerability assessments
Likelihood of severe outcomes decreased from the initial age-based host vulnerability assessment
A 70-year old individual who is healthy and without identified risk factors is planning to travel. The patient is adequately vaccinated, as per NACI recommendations.
This patient is at increased risk for severe COVID-19 if infected, by virtue of their age. They should be carefully screened for other risk factors. If none are identified, due to their vaccination status, consider reducing their host vulnerability assessment by one level from their age-based risk (moving them from high to moderate).
Likelihood of severe outcomes increased from the initial age-based host vulnerability assessment
Scenario 1. A 40-year old person with Down syndrome is planning to travel. The patient also has diabetes, and is adequately vaccinated, as per NACI recommendations.
This patient is at increased risk for severe COVID-19 if infected based on two identified underlying medical conditions. Given this, consider adjusting the host vulnerability assessment by two levels based on risk factors. However, as the individual is adequately protected by vaccine (which can reduce the vulnerability by one level), full adjustment will result in an increase of only one level, from moderate to high.
Scenario 2. A 50-year old individual with an allogeneic bone marrow transplant less than one year ago, and still receiving aggressive immune suppressive medication, is planning to travel. They are adequately vaccinated, as per NACI recommendations
This patient starts at moderate risk due to age. However, there is reason to assume that the individual is both at risk of severe disease due to major immune suppression, and lower immunogenicity of vaccination. Consider increasing the host vulnerability assessment by two levels due to the severity of the immune suppression, to very high and do not change the assessment based on vaccination status.
Among children, risk factors for severe disease include underlying medical conditions such as type 1 diabetes, neurological conditions, chronic pulmonary diseases (other than mild, controlled asthma), cardiac and circulatory congenital anomalies, obesity, and immune dysregulation associated with Down Syndrome and other immune compromising conditions Footnote 8 Footnote 12 ; many of which are the same as have been identified in adults (see Box 1 ) Footnote 2 Footnote 3 Footnote 4 . Hence, CATMAT recommends that the same approach (to increase the host vulnerability assessment level from the initial age-based assessment based on presence of other risk factors) be applied when assessing children. Some preventive therapies, such as antivirals or monoclonal antibodies, may not be indicated for use in children depending on age and weight requirements for eligibility. Expert opinion should be sought in guiding preventive pharmacologic options in vulnerable children.
Access to adequate medical care
Travellers, particularly those individuals deemed to be at moderate-to-very high risk of severe outcomes, who must travel, should also consider the availability and potentially high cost of adequate medical services in the destination country. In many circumstances, access to high quality specialized care will be more difficult to obtain. Further, any travel and treatment costs incurred as a result of COVID-19 might not be covered by medical travel insurance.
Travel itinerary and activities
The types of activities and actions that individuals undertake will influence the likelihood of exposure to SARS-CoV-2. In general, exposure likelihood increases with an increasing number of contacts, increasing duration of contact, decreasing proximity between individuals, and poor ventilation (i.e. indoor environments). Proper ventilation involves the replacement of indoor air with outdoor air, which helps to reduce the concentration of viral particles, or recirculating air through a high-efficiency particulate air (HEPA) filter Footnote 13 Footnote 14 .
For individuals who are considered to have high or very high host vulnerability, it is recommended that activities associated with an increased exposure likelihood be minimized (see Appendix ).
Vaccination outside of Canada
In general, CATMAT advises against receipt of vaccines not authorized in Canada, and/or offered in international locations. In some countries, it may be difficult to verify that vaccines are manufactured, stored, and administered under optimal conditions. Rather, emphasis should be placed on receiving vaccinations before leaving Canada.
In some circumstances, e.g., for long-term travellers and expatriates, vaccination outside of Canada might be unavoidable. If this is the case, then the traveller should be advised to opt for a vaccine product that is authorized for use by Health Canada and follow NACI guidance . If this is not possible, then preference is for vaccines that have met the necessary safety and efficacy criteria of the WHO Footnote 15 .
Serologic testing
Serologic testing for antibodies pre- or post-travel, as a surrogate for protection, is not recommended. Although commercial tests are available, they are of highly variable quality and accuracy. There is no adequately validated correlation between serologic results from a given test and risk of severe outcome.
The final host vulnerability assessment is comprised of the baseline assessment (based on the traveller's age, see Table 1), and any subsequent adjustments based on vaccination status (as per NACI guidance) and underlying medical conditions. Recommendations based on a traveller's final assessment are provided in Table 2. Additional considerations for very high risk travellers, specifically, are discussed below Table 2. The following broad guidance also applies to all individuals intending to travel outside of Canada:
- Eligible individuals should be adequately vaccinated against COVID-19, as per NACI guidance , while in Canada.
- Travellers should verify any travel requirements in place at their destination(s) and in Canada .
- Travellers should be familiar with Canadian recommendations for self-protection and be equipped to implement multiple individual public health measures based on their own preferences and any requirements at their destination .
Additional considerations for very high risk travellers
For individuals at very high risk for severe COVID-19, the following additional therapies may be considered, based on shared clinical decision making:
- Use of standby treatment with antiviral medication (eg: nirmatrelvir/ritonavir) in the absence of any drug-drug interactions, particularly where access to medical care and treatment may be limited, should the traveller become ill.
- CATMAT acknowledges that the availability of antiviral medication for this purpose may be limited at this time, as eligibility criteria across most jurisdictions require a positive test result and mild/moderate symptoms.
- Regarding monoclonal antibodies, any provincial or territorial recommendations for use of monoclonal antibodies may also apply to travellers.
- monoclonal antibodies have a continuously evolving role, and travellers should consult an expert to determine the most appropriate use of these agents for their individual situation and itinerary.
This statement was prepared by the COVID-19 Working Group: Libman M (Chair), Bui Y, Lagacé-Wiens P, Rossi C, Schofield S, Vaughan S, Tunis M and Jensen C (National Advisory Committee on Immunization Secretariat), and Farmanara N (CATMAT Secretariat) and was approved by CATMAT.
CATMAT would like to thank Andrea Boggild (member emeritus) for her contribution to earlier versions of the statement. CATMAT also acknowledges the technical and administrative support from the Centre for Border and Travel Health at the Public Health Agency of Canada for the development of this statement.
CATMAT members: Libman M (Chair), Acharya A, Bogoch I, Bui Y, Greenaway C, Lagacé-Wiens P, Lee J, Plewes K, Vaughan S,.
Liaison members: Angelo K (Centers for Disease Control and Prevention), Pernica J (Association of Medical Microbiology and Infectious Disease Canada), Viel-Thériault I (Canadian Paediatric Society).
Ex officio members: Marion D (Canadian Forces Health Services Centre, Department of National Defence), Rossi C (Medical Intelligence, Department of National Defence), Schofield S (Pest Management Entomology, Department of National Defence), and Zimmer R (Biologics and Radiopharmaceutical Drugs Directorate, Health Canada).
None declared.
The appendix below provides a non-exhaustive list of activities characterized by their anticipated SARS-CoV-2 exposure level. For those individuals who are considered at high or very high risk based on the host vulnerability assessment, one way to mitigate risk would be to modify activities to limit or reduce the risk of exposure.
Types of activities that are considered to be at relatively lower or higher likelihood of exposure to SARS-CoV-2
Lower likelihood.
Lower density outdoor environment. Distancing possible. Examples: Trekking in a wilderness area, playing golf, individual exercise outdoors, walking in uncrowded areas.
Medium likelihood
Lower density indoor activities. Distancing possible, perhaps improved ventilation (can include conveyances).
Examples: Shopping at a mall, attending a small language class, visiting an office building, purchasing groceries, travel on a major/modern airline/aircraft, travel in an uncrowded taxi with open windows, a tour on an uncrowded open-topped bus.
Higher density outdoor activities. Potentially limited distancing. Examples: Attending an open-air party/concert, visiting a crowded beach, swimming in a public pool, crowded open air bus tours, shopping at a crowded outdoor market, walking in a busy urban environment.
Higher likelihood
Higher density indoor activities. Often limited distancing, many people, limited ventilation (can include conveyances), actions that facilitate virus dispersal (e.g., singing, shouting, cheering, loud talking, exertion during exercise).
Examples: Travelling on a cruise ship, eating at a buffet, attending a large conference, attending a large indoor music concert, attending a sports event at an indoor stadium, attending a large indoor service, going to a busy indoor bar/restaurant, attending an indoor fitness class/crowded gym, travel in a crowded and closed and/or poorly ventilated conveyance (e.g., a local bus/minibus).
Canadian Public Health Association. Long acting monoclonal antibodies: Information for Canadians on COVID-19 pre-exposure prophylaxis (prevention) with monoclonal antibodies. 2022; Available at: https://www.cpha.ca/laab. Accessed August 18, 2022.
Return to footnote 1 referrer
Romero Starke K, Petereit-Haack G, Schubert M, Kämpf D, Schliebner A, Hegewald J, Seidler A. The age-related risk of severe outcomes due to COVID-19 infection: A rapid review, meta-analysis, and meta-regression. Int J Environ Res Public Health. 2020 Aug 17;17(16):5974. doi: https://doi.org/10.3390/ijerph17165974
Return to footnote 2 referrer
Gates M, Pillay J, Wingert A, Guitard S, Rahman S, Zakher B, et al. Risk factors associated with severe outcomes of COVID-19: An updated rapid review to inform national guidance on vaccine prioritization in Canada. medRxiv. 2021 May 22. doi: https://doi.org/10.1101/2021.04.23.21256014v2.
Return to footnote 3 referrer
Wingert A, Pillay J, Gates M, Guitard S, Rahman S, Beck A, et al. Risk factors for severe outcomes of COVID-19: a rapid review. medRxiv. 2020 Sep 1. doi: https://doi.org/10.1101/2020.08.27.20183434.
Return to footnote 4 referrer
Smith C, Odd D, Harwood R, Ward J, Linney M, Clark M, et al. Deaths in children and young people in England following SARS-CoV-2 infection during the first pandemic year: a national study using linked mandatory child death reporting data. medRxiv, 2021 July 7. doi: https://doi.org/10.1101/2021.07.07.21259779
Return to footnote 5 referrer
Ward J, Harwood R, Smith C, Kenny SE, Clark M, Davis PJ, et al. Risk factors for intensive care admission and death amongst children and young people admitted to hospital with COVID-19 and PIMS-TS in England during the first pandemic year. medRxiv. 2021 Jan 1. doi: https://doi.org/10.1101/2021.07.01.21259785
Return to footnote 6 referrer
Harwood R, Yan H, Da Camara NT, Smith C, Ward J, Tudur-Smith C, et al. Which children and young people are at higher risk of severe disease and death after SARS-CoV-2 infection: a systematic review and individual patient meta-analysis. medRxiv. 2021 Jan 1. doi: https://doi.org/10.1101/2021.06.30.21259763
Return to footnote 7 referrer
Kompaniyets L, Agathis NT, Nelson JM, Preston LE, Ko JY, Belay B, et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA network open. 2021 Jun 1;4(6):e2111182. doi: https://doi.org/10.1001/jamanetworkopen.2021.11182
Return to footnote 8 referrer
Kompaniyets L, Pennington AF, Goodman AB, Rosenblum HG, Belay B, Ko JY, et al. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020–March 2021. Prev Chronic Dis 2021;18:210123. DOI: http://dx.doi.org/10.5888/pcd18.210123
Return to footnote 9 referrer
Centers for Disease Control and Prevention. Scientific brief: Evidence used to update the list of underlying medical conditions associated with higher risk for severe COVID-19. 2022; Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html. Accessed: July 07, 2022.
Return to footnote 10 referrer
National Advisory Committee on Immunization. Canadian Immunization Guide. COVID-19 vaccine (chapter). Ottawa (ON): PHAC; 2022. Available at: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-26-covid-19-vaccine.html
Return to footnote 11 referrer
Choi JH, Choi SH, Yun KW. Risk ractors for severe COVID-19 in children: A systematic review and meta-analysis. J Korean Med Sci. 2022 Feb 7;37(5):e35. doi: 10.3346/jkms.2022.37.e35
Return to footnote 12 referrer
Government of Canada. COVID-19: Improving indoor ventilation. 2021; Available at: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/prevention-risks/covid-19-improving-indoor-ventilation.html. Accessed: July 07, 2022.
Return to footnote 13 referrer
Centers for Disease Control and Prevention. Ventilation in Buildings. 2021; Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/ventilation.html?s=09. Accessed July 10, 2021.
Return to footnote 14 referrer
World Health Organization. Regulation and prequalification, emergency use listing: COVID-19 vaccines. 2021; Available at: https://www.who.int/teams/regulation-prequalification/eul/covid-19. Accessed July 07, 2022.
Return to footnote 15 referrer
Page details
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Importation
- Bringing an Animal into the U.S.
- Bringing a Dog into the U.S.
- Bringing Animal Products into the U.S.
- Bringing Human Remains into the U.S.
- Laws and Regulations
- Documents for Veterinarians to Complete for Importing a Dog into the U.S.
- Information for Airlines on Dogs Being Imported into the U.S.
- View All Home
Entry Requirements for Dogs from Dog-Rabies Free or Low-Risk Countries
At a glance.
The one form you will need is the CDC Dog Import Form if your dog has been only in countries that are dog rabies-free or low-risk in the 6 months before entering or returning to the U.S. This page outlines the process and requirements.
Recent Changes
CDC values the feedback received from various countries, industry partners, and the public. CDC simplified the process for meeting requirements for dogs arriving from dog rabies-free or low-risk countries.
Starting on August 1, 2024, the only required documentation for dogs entering or returning to the United States that have been only in dog rabies-free or low-risk countries in the past 6 months is the CDC Dog Import Form .
Dog Rabies-free or Low-risk Countries
Cdc dog import form.
For dogs that have been only in dog rabies-free or low-risk countries in the 6 months before U.S. entry, this is the only form that is needed.
CDC Dog Import Form
How long is the form's receipt valid.
The receipt is valid for 6 months from when it’s issued unless the dog visits a high-risk country or a different dog rabies-free or low-risk country during that time. For example, if the dog travels frequently between the U.S. and Canada, the same form can be used for travel from Canada until the expiration date listed on the CDC Dog Import Form receipt. If the dog typically travels between Canada and the U.S. but you take another trip between the U.S. and France, you will need a new CDC Dog Import Form receipt listing France as the country of departure.
Can I use the form's receipt multiple times?
The receipt can be used for multiple entries into the United States as long as the dog has not been in a high-risk country in the past 6 months. If the dog visits a different dog rabies-free or low-risk country than the one listed as the country of departure on the form, you should complete a new CDC Dog Import Form listing that country as the country of departure. The country of departure listed on your receipt should match the country your dog is departing from to come into the U.S.
Who completes the form?
This form should be completed by the person importing (bringing) the dog into the United States (the importer, owner, or shipper). If you are completing the form on behalf of an importer who has difficulty completing this form, read the statements aloud to the importer and confirm they understand the information provided and agree to the Terms of Acceptance.
How much does it cost?
How many dogs can be included on one form.
Each dog must have its own form. If you are bringing more than one dog to the U.S., you will need to complete a separate form for each dog.
When should the form be completed?
You can fill out the form on the day of travel. We recommend completing it a few days or up to six months before travel from a dog rabies-free or low-risk country, just to be prepared.
How do I show the form's receipt to officials?
CDC Dog Import Form receipt can be printed or shown on a phone screen to U.S. customs officials or airlines (if applicable).
What else is required for dogs from dog rabies-free or low-risk countries?
In addition to having a receipt for a CDC Dog Import Form :
- Dogs must appear healthy upon arrival.
- Dogs must be at least 6 months old at time of entry or return to the U.S.
- Dogs must have a microchip that can be detected with a universal scanner to identify them.
Where the Dog Can Arrive
Questions.
CDC regulations govern the importation of animals and animal products capable of causing human disease.
For Everyone
Health care providers, public health.
Confused about proof-of-vaccination requirements for travel? Your questions answered
The federal government's requirement for proof-of-vaccination to travel domestically starts oct. 30.
Social Sharing
This week, the federal government announced that as of Oct. 30 , all travellers boarding a plane, train or marine vessel in Canada will need proof of full vaccination against COVID-19.
"Fully vaccinated" is defined as a full series of a Health Canada-approved vaccine, with the last dose having been administered at least 14 days prior to the day of travel. A combination of approved shots is also acceptable.
There will be a period of transition through November for people who are in the process of getting fully vaccinated, but eventually, to travel by air, rail or water in Canada, or from Canada to international destinations on Canadian airlines, full vaccination will be required.
That prompted some questions from you.
Will the government allow a 3rd shot for those who have mixed vaccines so they can fulfil November travel plans?
For domestic travel, your two mixed doses of Health Canada-approved vaccines are fine and entirely acceptable.
Travelling internationally is not as straightforward, as countries have their own specific rules with respect to entry, vaccination status and whether a quarantine is required.
Right now, the Canadian government still advises against any unessential travel outside of Canada. But many Canadians have travelled to the U.S., which sometime in November will have its own requirement for entrants to be fully vaccinated.
The U.S. Centers for Disease Control and Prevention (CDC) announced late Friday that the United States will accept international visitors inoculated with COVID -19 vaccines authorized by U.S. regulators or the World Health Organization , which includes vaccines that were administered in Canada but not in the U.S., such as Oxford-AstraZeneca.
While it's unclear whether that includes mixed doses, the CDC said it would release "additional guidance and information as the travel requirements are finalized."
The National Advisory Committee on Immunization (NACI) has not yet recommended a third shot simply for the ability of international travel.
But some provinces have moved ahead on that front. Saskatchewan , Alberta and Quebec announced in the summer that they would offer a third shot to people whose vaccines weren't recognized internationally.
This week, Manitoba announced it would also offer a 3rd shot of an mRNA vaccine to anyone who got a full mixed series, or two doses of AstraZeneca, or one dose of Janssen.
"We don't recommend a booster shot for international travel," Dr. Joss Reimer, medical lead of Manitoba's vaccine task force said during a briefing Wednesday. "What we did instead is just make a third dose available for travellers where they are not allowed to go to certain destinations because that destination doesn't recognize what they received here."
- Fully vaccinated and ready to travel abroad? You might still face hurdles
Dr. Theresa Tam, Canada's chief public health officer, said late last month that the government continues to engage with other countries about accepting a mixed vaccine dose; those talks are still ongoing.
It is up to any individual country to decide what qualifies as "fully vaccinated" when it comes to allowing foreigners to enter.
"We've been presenting our data, for example, in the vaccine effectiveness of the mixed dose schedule such as AstraZeneca followed by mRNA vaccines," Tam said Sept. 24 in Ottawa.
- U.S. vaccination requirement for air passengers worries Canadians with mixed vaccines
- Biden easing foreign air travel restrictions, requiring vaccines
She added that progress may be quicker when it comes to European countries because some of them have also used the mixed-dosing schedules.
"But we still have to advise travellers that they must check in with the specific country requirements prior to travel because it is a bit of a varied landscape out there," she said.
Will the new rules mean that we won't need a negative COVID-19 test three days before a flight within Canada anymore?
There will be a short period of time when individuals who are in the process of being vaccinated will be able to travel domestically if they can show a negative COVID-19 molecular test within 72 hours of their travel date.
But that period will end Nov. 30.
After that, showing a negative COVID-19 test to travel within Canada — rather than proof-of-vaccination — will no longer be an option.
"By the end of November, if you're 12 or older and want to fly or take the train, you'll have to be fully vaccinated as will staff," said Prime Minister Justin Trudeau, during a briefing Wednesday on the new rules. "Testing will no longer be an option before boarding."
For international travel, the rules around pre-flight testing remain unchanged .
International travellers coming to Canada still need to show a negative molecular COVID-19 test that was done within 72 hours of their flight departure time. Rapid antigen tests are not accepted.
WATCH | Justin Trudeau lays out the new proof-of-vaccination rules:

Prime Minister Justin Trudeau announces new vaccine mandate
I got a vaccine not approved in canada. can i get another set of vaccines so i can travel .
Eligibility for a new set of vaccines is at the discretion of each province or territory, as they are responsible for vaccine eligibility and distribution.
Many do currently offer an additional shot to individuals already vaccinated against COVID-19 with a vaccine not approved by Health Canada, including Manitoba , Ontario , and Nova Scotia .
It is recommended that anyone who received a COVID-19 vaccine not approved in Canada contact their local health authority for information about getting a Health Canada-approved vaccine.

What about people who have recovered from COVID?
Recovery from COVID-19 is unlikely to get you an exemption from the need to be fully vaccinated to travel domestically.
"For the vast, vast majority of people, the rules are very simple — to travel, you've got to be vaccinated," Trudeau said during the same briefing.
"If you haven't gotten your shots yet but want to travel this winter, let me be clear, there will only be a few extremely narrow exceptions, like a valid medical condition."
He said the government is working with Health Canada to define those exemptions, but warned they will be onerous to obtain.
"Let me say that simply having a personal conviction that vaccines are bad will not be nearly enough to qualify for an exemption."
Right now, the government does not recognize travellers to Canada who have recovered from COVID-19 and had only one dose of a vaccine to be "fully vaccinated."
According to its information page on travel , "If you have recovered from COVID-19, you still need a full series of an accepted COVID-19 vaccine or combination of accepted vaccines."
WATCH | Are these new rules legal?

Are the government's new travel rules legal?
How long will these measures will be put in place .
The government did not say. It will be up to the federal government to lift any national COVID-19 restrictions or regulations.
Clarifications
- B.C. has been taken off the list of provinces that offer third doses of COVID-19 vaccines so people can travel. Third doses are not available to the general population for travel in the province. Oct 13, 2021 11:59 AM ET
Related Stories
- Federal public servants, RCMP and air and rail travellers must be vaccinated by month's end, Trudeau says
Advertisement
Supported by
What to Know About the C.D.C. Guidelines on Vaccinated Travel
In updated recommendations, the federal health agency said both domestic and international travel was low risk for fully vaccinated Americans. But travel remains far from simple.
- Share full article

By Ceylan Yeginsu
The Centers for Disease Control and Prevention updated its guidance for fully vaccinated Americans in April, saying that traveling both domestically and internationally was low risk.
The long-awaited recommendations were issued by federal health officials after a series of studies found that vaccines administered in the United States were robustly effective in preventing infections in real-life conditions.
One is considered fully vaccinated two weeks after receiving the single dose of the Johnson & Johnson vaccine, or two weeks after receiving the second dose of the Pfizer-BioNTech or Moderna shots.
If you decide to travel, you might still have some questions. Here are the answers.

Will I still need to wear a mask and socially distance while traveling?
Yes. Under federal law, masks must be worn at airports in the United States, onboard domestic flights and in all transport hubs. The C.D.C. says that as long as coronavirus measures are taken in these scenarios, including mask wearing, fully vaccinated Americans can travel domestically without having to take a test or quarantine, although the agency warns that some states and territories may keep their local travel restrictions and recommendations in place.
For those wishing to travel internationally, a coronavirus test will not be required before departure from the United States unless mandated by the government of their destination. Vaccinated travelers are still required to get tested three days before travel by air into the United States, and are advised to take a test three to five days after their return, but will not need to self-quarantine.
Can I go abroad?
Yes, but only to countries that will have you.
More than half the world’s countries have reopened to tourists from the United States, including the countries of the European Union , which on June 18 added the United States to its “safe list” of countries, meaning that American travelers can now visit. While the European Union aims to take a coordinated approach to travel this summer, member states will be allowed to set their own requirements for travelers from individual countries based on their own epidemiological criteria, which means they may require testing or vaccination.
Some places like Turkey, Croatia and Montenegro had already been welcoming Americans with negative test results. Greece joined that growing list in May, ahead of most European countries, opening to fully vaccinated tourists and other foreigners with a negative test.
Many Caribbean nations have reopened to American tourists, but each has its own coronavirus protocols and entry requirements.
Here’s a full list of countries Americans can currently travel to.
What about domestic travel? Is it free and clear to cross state borders?
If you are fully vaccinated, the C.D.C. says you can travel freely within the United States and that you do not need to get tested, or self-quarantine, before or after traveling. But some states and local governments may choose to keep travel restrictions in place, including testing, quarantine and stay-at-home orders. Hawaii , for instance, still has travel restrictions in place.
Before you travel across state lines, check the current rules at your destination.
How are they going to check that I’m fully vaccinated?
Right now, the best way to prove that you have been vaccinated is to show your vaccine card .
Digital vaccine and health certificates showing that people have been vaccinated or tested are in various stages of development around the world and are expected, eventually, to be widely used to speed up travel.
The subject of “ vaccine passports ” is currently one of the most hotly debated topics within the travel industry, with questions over the equity of their use and concerns over health and data privacy.
In early April, Gov. Ron DeSantis of Florida issued an executive order that would ban local governments and state businesses from requiring proof of vaccination for services.
And in March, the European Union endorsed its own vaccine certificate , which some countries are already using, with more expected to adopt it by July 1.
But what about my kids? What’s the guidance on traveling with unvaccinated people?
The C.D.C. advises people against travel unless they have been vaccinated. If you must travel, the agency recommends testing one to three days before a trip and following all coronavirus guidance at your destination.
In May, the F.D.A. expanded its emergency use authorization of the Pfizer-BioNTech coronavirus vaccine to include adolescents between 12 and 15 years of age.
All air passengers aged two and older coming into the United States, including fully vaccinated people, are required to have a negative Covid-19 test result taken no more than three days before they board their flight.
What is my moral obligation to the places I visit where most people are not vaccinated?
The United States inoculation rollout has been among the fastest in the world, but there is a stark gap between its rapid rollout and the vaccination programs in different countries. Some nations have yet to report a single dose being administered.
Many countries are currently seeing a surge in new cases and are implementing strict coronavirus protocols, including mask mandates in public spaces, capacity limits at restaurants and tourist sites and other lockdown restrictions.
It is important to check coronavirus case rates, measures and medical infrastructure before traveling to your destination and not to let your guard down when you get there. Even though you are fully vaccinated, you may still be able to transmit the disease to local communities who have not yet been inoculated.
You can track coronavirus vaccination rollouts around the world here.
Follow New York Times Travel on Instagram , Twitter and Facebook . And sign up for our weekly Travel Dispatch newsletter to receive expert tips on traveling smarter and inspiration for your next vacation.
Ceylan Yeginsu is a London-based reporter. She joined The Times in 2013, and was previously a correspondent in Turkey covering politics, the migrant crisis, the Kurdish conflict, and the rise of Islamic State extremism in Syria and the region. More about Ceylan Yeginsu
- Skip to main content
- Skip to site information
Language selection
Help us to improve our website. Take our survey !
Travel outside Canada
It is important to be prepared and to expect the unexpected wherever in the world you may be. Here is helpful information on health and safety, travel documents, Canada-U.S border wait times, travelling with children and more.

New rules for dogs entering the U.S
New entry requirements for dogs entering the United States from Canada will take effect on August 1, 2024:
- Dogs travelling to the U.S
Services and information
Travel advice and advisories.
Official Government of Canada travel information
Travel Advice and Advisories archives
Government of Canada’s Travel Advice and Advisories archives from November 16, 2012 to the present
Health and safety outside Canada
Essential information on travel health and safety risks and how to prevent them
Travel documents
Passports, visas, international driving permits and other documents you will need when you travel abroad
Types of travellers
Find travel information specific to dual citizens, women, older people, 2SLGBTQI+ people and other types of travellers
Canada to U.S. border wait times
Regularly updated list of wait times at border crossing points for drivers going to the U.S. from Canada
- Registration of Canadians Abroad
Sign up with the Registration of Canadians Abroad service to stay connected to Canada in case of an emergency abroad or an emergency at home
Children and travel
Everything you need to know to travel safely with children abroad
Living abroad
Be informed and prepared for the benefits and challenges of working, studying, retiring, volunteering or travelling on business abroad before you leave Canada
Resources for educators, students and travel counsellors
Online training and web-based resources for educators, students and travel counsellors
Publications
Ordering and reading our wide range of publications is a good first step to a safe and healthy trip abroad
Cannabis and international travel
Understand and avoid the risks related to cannabis and international travel
Travelling and money
Information about using cash, credit cards and debit cards abroad, taxation and travelling with $10,000 or more
Schengen Area
Important information for travellers to Europe
What we are doing
Policies, acts and regulations.
- Citizenship Act
- Canadian Air Transport Security Authority Act
- Department of Foreign Affairs, Trade and Development Act
- Public Health Agency of Canada Act
- Bon voyage, but... Essential information for Canadian travellers
- Travelling with children
- Well on Your Way - A Canadian's Guide to Healthy Travel Abroad
All related publications
- CATSA claims forms
- Recommended consent letter for children travelling abroad

Before travelling, talk to a health care provider about your travel plans and how you can reduce your risk of getting viruses like mpox.

A quick searchable guide to what you can and cannot bring on a plane.

A free service provided by Global Affairs Canada that keeps you connected to Canada in case of an emergency abroad or at home.
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 30, Number 10—October 2024
Pathogenicity of Highly Pathogenic Avian Influenza A(H5N1) Viruses Isolated from Cats in Mice and Ferrets, South Korea, 2023
Suggested citation for this article
The prevalence of highly pathogenic avian influenza (HPAI) A(H5N1) viruses has increased in wild birds and poultry worldwide, and concomitant outbreaks in mammals have occurred. During 2023, outbreaks of HPAI H5N1 virus infections were reported in cats in South Korea. The H5N1 clade 2.3.4.4b viruses isolated from 2 cats harbored mutations in the polymerase basic protein 2 gene encoding single amino acid substitutions E627K or D701N, which are associated with virus adaptation in mammals. Hence, we analyzed the pathogenicity and transmission of the cat-derived H5N1 viruses in other mammals. Both isolates caused fatal infections in mice and ferrets. We observed contact infections between ferrets, confirming the viruses had high pathogenicity and transmission in mammals. Most HPAI H5N1 virus infections in humans have occurred through direct contact with poultry or a contaminated environment. Therefore, One Health surveillance of mammals, wild birds, and poultry is needed to prevent potential zoonotic threats.
Since the emergence of the highly pathogenic avian influenza (HPAI) A(H5N1) virus (A/chicken/Scotland/59) in Scotland, UK, several outbreaks of H5Nx viruses have been reported in poultry worldwide ( 1 ). In 1996, an HPAI H5N1 virus, A/goose/Guangdong/1/1996 (Gs/GD), was identified, and the Gs/GD lineage H5 viruses have been circulating in poultry and wild aquatic bird reservoirs for >25 years ( 1 , 2 ). HPAI H5N1 viruses pose a global threat to the poultry industry and public health because of frequent outbreaks in chicken, ducks, and other poultry ( 3 ). According to the World Health Organization, 882 cases of avian influenza A(H5N1) infections in humans have been reported globally from January 1, 2003, to December 21, 2023, resulting in 461 deaths (52% mortality rate) ( 4 ).
Since 2005, HPAI H5N1 viruses have diversified genetically, forming numerous genotypes through reassortment with other avian influenza A viruses ( 5 ). HPAI H5N1 clade 2.3.4.4b viruses of the Gs/GD lineage emerged in Europe in 2020, causing outbreaks in wild birds and poultry in many countries ( 5 ). The spread of clade 2.3.4.4b viruses was reported in 26 countries worldwide; the virus infected >48 mammal species ( 2 , 5 – 7 ). In 2022, mass deaths of >20,000 sea lions from HPAI H5N1 infections were confirmed along the coast of South America, including coastal Peru, Chile, Argentina, Uruguay, and Brazil. In addition, in 2023, unusual deaths of cats were reported in Poland ( 8 – 10 ). Therefore, concerns about the risk for interspecies transmission and human-to-human spread of H5N1 viruses have been growing because of the acquisition of interhost transmission capability and the increase in HPAI H5N1 viruses found in mammals ( 11 ).
In South Korea, HPAI H5N1 clade 2.3.4.4.b viruses were identified in wild birds in 2021, which was followed by infection outbreaks in poultry farms ( 12 ). During autumn 2022, introductions of > 2 types of HPAI H5N1 clade 2.3.4.4b viruses that originated from Eurasian breeding grounds and North America occurred simultaneously, and various genotypes were subsequently detected in wild birds and domestic poultry ( 13 , 14 ). During July 2023, unusual deaths of cats at animal shelters occurred in the Yongsan and Gwanak Districts of Seoul, South Korea, caused by HPAI H5N1 viruses ( 15 , 16 ); viruses isolated from cats were obtained from each animal shelter. We analyzed the pathogenicity and transmission characteristics of 2 cat-derived virus isolates by using molecular methods and by conducting experiments in mouse and ferret infection models. We performed all animal experiments in strict accordance with general animal care guidelines mandated under the Guidelines for Animal Use and Care of the Korea Disease Control and Prevention Agency (KDCA).
Materials and Methods
We grew and maintained MDCK cells (American Type Culture Collection, https://www.atcc.org ) in Eagle’s Minimum Essential Medium (WELGENE, https://www.welgene.com ) containing 5% fetal bovine serum, 1 mmol/L L-glutamine, and penicillin/streptomycin (Thermo Fisher Scientific, https://www.thermofisher.com ). We incubated the cells at 37°C in 5% CO 2 until use.
Virus Distribution
The Animal and Plant Quarantine Agency (APQA), South Korea, provided 3 HPAI H5N1 virus isolates and deposited their whole-genome sequences in the GISAID EpiFlu database ( http://www.gisaid.org ). We propagated the isolates in specific pathogen-free embryonated chicken eggs (second passage) and confirmed that their sequences were identical to those provided by the APQA ( Table 1 ). A/duck/Korea/H493/2022(H5N1) (GISAID accession no. EPI_ISL_15647834) originated from a duck farm in the Yecheon area in October 2022. A/feline/Korea/M302–6/2023(H5N1) (accession no. EPI_ISL_18819809) was from the Yongsan District, and A/feline/Korea/M305–7/2023(H5N1) (accession no. EPI_ISL_18819807) was from the Gwanak District; both of those viruses from cats were obtained from animal shelters during July 2023.
Genetic and Phylogenetic Analysis
We extracted virus RNA by using the RNeasy Mini Kit (QIAGEN, https://www.qiagen.com ) and performed gene amplification and library preparation by using the Illumina Microbial Amplicon Prep-Influenza A/B kit (Illumina, https://www.illumina.com ). Subsequently, we sequenced whole genomes of the viruses on a MiSeq instrument by using MiSeq Reagent Kit v2 (Illumina) to obtain 2 × 150-bp read lengths. For phylogenetic analysis, we searched for sequences, other than those analyzed in this study, in the GISAID database. We inferred phylogenetic relationships of sequences obtained in this study by using the maximum-likelihood method, 1,000 bootstrap values, and MEGA 7 software ( 17 ).
Virus Titrations
We determined virus titers of oropharyngeal and cloacal swab samples, nasal washes, and homogenized tissue samples by performing endpoint titrations in MDCK cell monolayers. We inoculated MDCK cells with 10-fold serial dilutions of each sample prepared in fetal bovine serum–free medium containing L-1-tosylamido-2-phenylethyl chloromethyl ketone–treated trypsin and penicillin/streptomycin. After a 72-hour incubation at 37°C, we detected viruses in a standard hemagglutination assay by using 0.5% turkey erythrocytes. We expressed mean virus titers as log 10 50% tissue culture infectious dose (TCID 50 ). The detection limit was 0.5 log 10 TCID 50 /mL. We estimated virus titers by using t -tests and 2-way analysis of variance in GraphPad Prism 9 (GraphPad Software Inc., https://www.graphpad.com ).
Neuraminidase Inhibitor Resistance
We used the neuraminidase (NA) inhibitors oseltamivir and peramivir (Cayman Chemical, https://www.caymanchem.com ) and zanamivir (Sigma-Aldrich, https://www.sigmaaldrich.com ) to assess drug susceptibility of the 3 virus isolates. We used a fluorescence assay containing the 2′-(4-methylumbelliferyl)-α-D- N -acetylneuraminic acid substrate (Sigma-Aldrich) ( 18 , 19 ). We normalized influenza viruses to equivalent NA activities and incubated virus samples with 10-fold serial dilutions (0–30,000 nmol/L) of oseltamivir, zanamivir, or peramivir. We measured the fluorescence signal by using a Mithras LB 940 reader (Berthold Technologies, https://www.berthold.com ) at excitation/emission wavelengths of 355/460 nm. We estimated the 50% inhibitory concentration (IC 50 ) for each sample from dose-response curves by using the sigmoidal, 4-parameter, logistic nonlinear regression equation in GraphPad Prism 9. To assess neuraminidase inhibitor (NAI) resistance, we divided the IC 50 value of the virus being analyzed by the IC 50 value of the NAI-sensitive influenza A(H1N1)pdm09 virus strain, which has the amino acid H274 in neuraminidase, making it NAI-susceptible.
Experimental Infections of Mice and Ferrets
We anesthetized groups of 6-week-old BALB/c mice (SAMTAKO, http://www.samtako.com ) (n = 5/group) with ketamine and intranasally inoculated them with 50 µL of 10 0 –10 6 TCID 50 /mL of virus. After virus inoculation, we weighed the mice and monitored them for clinical signs and death for 14 days. For virus replication studies, we intranasally inoculated 15 mice per group with 50 µL of 10 3 50% median lethal dose (MLD 50 )/mL. We euthanized 5 mice per group on days 3, 5, and 7 postinoculation and assessed virus titers in brain, trachea, nasal turbinate, lung, heart, liver, kidney, spleen, and intestinal samples.
We anesthetized 20–22-week-old ferrets (IDBio, http://www.idbio.co.kr ) (n = 12/group) with ketamine and intranasally inoculated them with 1 mL of 10 3 MLD 50 /mL of virus. After virus inoculation, we weighed and monitored 3 ferrets per virus group for clinical signs and death for 14 days. We used the remaining 9 ferrets per group for virus replication studies. We euthanized 3 ferrets per virus group on days 3, 5, and 7 postinoculation and assessed virus titers in brain, trachea, nasal turbinate, lung, heart, liver, kidney, spleen, and intestinal samples. To assess virus transmission via contact infection, we intranasally inoculated 1 ferret (per virus) with 1 mL of 10 3 MLD 50 /mL virus and then housed serologically-naive ferrets (n = 2) in the same cage the next day (1 cage/virus). We collected nasal wash samples from each ferret on days 3, 5, and 7 postinoculation and measured virus titers. We euthanized mice and ferrets showing >20% body weight loss, which we considered a humane endpoint.
Serologic Tests
We collected blood samples from ferrets in the infection groups 14 days postinfection and in the transmission groups 14 days after contact with a virus-infected ferret. We determined seroconversion by using a microneutralization assay.
Genetic Characterization of H5N1 Viruses Isolated from Cats
The Korea Disease Control and Prevention Agency (KDCA) received 2 different HPAI H5N1 viruses from cats in animal shelters that were collected by APQA, which we used to characterize infections in other mammals. We used the 2 H5N1 viruses, A/feline/Korea/M302–6/2023(H5N1) from Yongsan (abbreviated as YS/2023) and A/feline/Korea/M305–7/2023(H5N1) from Gwanak (abbreviated as GA/2023), to determine how the pathogenicity and transmission of those H5N1 viruses differed from previously prevalent H5N1 viruses. We also analyzed the virus isolated from a duck, A/duck/Korea/H493/2022(H5N1) (abbreviated as YC/2022), representing the first poultry outbreak in autumn 2022.
We conducted whole-genome sequence analysis to confirm genetic characteristics of the YC/2022, YS/2023, and GA/2023 viruses and observed polybasic residues (REKRRKR/GLF) within the cleavage sites of hemagglutinin (HA), classifying all 3 viruses as HPAI. Phylogenetic analysis confirmed the viruses belonged to clade 2.3.4.4b ( Table 1 ; Appendix Figure). YS/2023 and GA/2023 viruses shared 99.9%–100% genetic similarity ( Appendix Table). They also showed close genetic relatedness to H5N1 viruses that have been circulating in wild birds and poultry in Asia since 2022, including in South Korea, China, and Japan ( 15 ).
We identified amino acid substitutions related to mammal adaptation in both YS/2023 and GA/2023 viruses. In both viruses, we found mutations S123P, S133A, and T156A (H5 numbering), which enhance binding affinity of the HA protein to α2,6-sialic acid on the host cell surface and contribute to increased mammal receptor tropism ( 20 ; J. Yang et al., unpub. data, https://doi.org/10.1101/2024.07.09.602706 ). In addition, in the polymerase basic 2 (PB2) gene segment, we identified mutations encoding D701N in YS/2023 and E627K in GA/2023; both substitutions are mammal-adapting mutations known to increase polymerase activity and virulence in mammals ( Table 2 ) ( 21 – 23 ). However, mutations associated with antiviral drug resistance, such as H274Y in NA and S31N in the matrix protein, were not detected ( 21 , 24 ).
Virus Pathogenesis in a Mouse Model

Figure 1 . Survival of mice infected with highly pathogenic avian influenza A(H5N1) viruses isolated from cats in South Korea, 2023. Viruses were isolated from 2 cats and 1 duck. A) A/feline/Korea/M302-6/2023; B)...
We intranasally inoculated 10-fold serial dilutions of infectious dose for each virus into 6-week-old BALB/c mice. After a 2-week observation period, the MLD 50 values were 10 1.5 TCID 50 /mL for YS/2023 and 10 0.5 TCID 50 /mL for GA/2023, whereas the MLD 50 was 10 4.8 TCID 50 /mL for YC/2022. The 2 viruses isolated from cats had ≈10-fold difference in MLD 50 values between them, but their MLD 50 values were >1,000-fold lower than that of the duck isolate ( Table 1 , Figure 1 ).
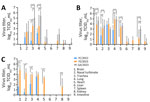
Figure 2 . Virus titers in organs of mice infected with highly pathogenic avian influenza A(H5N1) viruses isolated from cats in South Korea, 2023. Viruses were isolated from 2 cats (YS/2023 and GA/2023)...
To assess detailed clinical symptoms and virus replication in internal organs, we intranasally inoculated 50 µL of 10 3 MLD 50 /mL (YS/2023, 10 4.5 TCID 50 /mL; GA/2023, 10 3.5 TCID 50 /mL; YC/2022, 10 7.8 TCID 50 /mL) of each virus into 6-week-old BALB/c mice (n = 15 in each group). All infected mice exhibited clinical symptoms, such as weight loss, ruffled fur, lethargy, and ataxia, within 5 days postinfection. Virus infection was confirmed in the respiratory tract of all mice on day 3 postinfection, and only viruses isolated from cats were detected in all organs (including the brain) by day 5 postinfection ( Figure 2 ). Furthermore, all virus-infected mice had virus titers in lung, trachea, and nasal turbinate samples beginning on day 3 during the early stage of infection ( Figure 2 ). By day 5, only mice infected with both cat isolates (YS/2023 and GA/2023) had virus titers in 9 organs; we observed high virus titers in brain, nasal turbinate, trachea, lung, and heart samples. In particular, mice infected with GA/2023 exhibited high titers in lung (10 5.4 TCID 50 /mL) and trachea (10 5.1 TCID 50 /mL) tissue on day 3 and in lung (10 4.9 TCID 50 /mL) and brain (10 4.7 TCID 50 /mL) tissue on day 5, but all mice died before day 7. Mice infected with YS/2023 had high titers (10 3.9 –10 4.9 TCID 50 /mL) in lung, trachea, and brain tissues on day 5 and in brain (10 4.8 TCID 50 /mL) and nasal turbinate (10 4.25 TCID 50 /mL) tissue on day 7. Both cat-derived virus isolates used to infect mice showed a systemic infection pattern and high lethality; high virus titers occurred in most organs, including the brain. The duck isolate, YC/2022, was detected only in the brain and respiratory organs (nasal turbinate, trachea, and lungs) by day 7; virus titers were lower than those for the cat isolates ( Figure 2 ).
Virus Pathogenesis and Transmission in a Ferret Model

Figure 3 . Survival of ferrets infected with highly pathogenic avian influenza A(H5N1) viruses isolated from cats in South Korea, 2023. Viruses were isolated from 1 duck (YC/2022) and 2 cats (YS/2023 and...

Figure 4 . Virus titers in organs of ferrets infected with highly pathogenic avian influenza A(H5N1) viruses isolated from cats in South Korea, 2023. Viruses were isolated from 1 duck (YC/2022) and 2...
Ferrets intranasally infected with the 2 cat-derived H5N1 virus isolates showed severe clinical symptoms, including sneezing, nasal discharge, diarrhea, and neurologic complications. They also exhibited a mean peak reduction in bodyweight of 5.5%–25.7% and fever of 0.7°C–2.6°C above baseline temperature. All ferrets died by day 8 in the GA/2023 infection group and by day 9 in the YS/2023 group (100% mortality rate) ( Figure 3 ). The ferrets infected with the GA/2023 and YS/2023 viruses showed a systemic infection pattern. In ferrets infected with GA/2023, the virus was detected in all organs except the kidneys by day 5, and virus titers of 10 0.8 –10 2.9 TCID 50 /mL were detected in the brain and respiratory organs. Those titers were lower than titers observed in ferrets infected with YS/2023 (10 2.3 –10 3.4 TCID 50 /mL) that survived longer ( Figure 4 ). The ferrets infected with YC/2022 showed the highest virus titers in the respiratory tract (10 2.3 –10 5.4 TCID 50 /mL) until day 5, and infection was observed in all organs. However, 1 ferret died in the YC/2022 group on day 11, resulting in a survival rate of 66.6% ( Figure 3 ).

Figure 5 . Survival rates after ferret-to-ferret contact transmission in study of pathogenicity of highly pathogenic avian influenza A(H5N1) viruses isolated from cats, South Korea, 2023. Viruses were isolated from 2 cats and...

Figure 6 . Virus titers after ferret-to-ferret contact transmission in study of pathogenicity of highly pathogenic avian influenza A(H5N1) viruses isolated from cats, South Korea, 2023. Virus titers were measured in nasal washes...
To assess virus transmission via contact with YS/2023, GA/2023, and YC/2022, each virus was intranasally inoculated into 1 ferret and 2 serologically naive ferrets were moved into the same cage as the infected animal. The ferrets inoculated with either cat-derived virus died on day 8 postinoculation. In contrast, only 1 of 2 ferrets in contact with the YS/2023-infected ferret died on day 13; no seroconversion was observed in the surviving ferret. The 2 ferrets in contact with the GA/2023-infected ferret died by day 9 (100% mortality rate) ( Figure 5 ). Virus concentrations increased (10 2.3 –10 5.5 TCID 50 /mL) in nasal wash samples collected from ferrets exposed to the cat-derived viruses on days 3, 5, and 7, confirming transmission and infection in naive ferrets through contact with infected animals ( Figure 6 ). In contrast, the ferret infected intranasally with YC/2022 did not die, although the virus was detected in nasal washes. Both ferrets in that contact group survived; no virus was detected in nasal washes and no seroconversion was observed, inferring that contact transmission of YC/2022 did not occur.
Antiviral Drug Susceptibility of Influenza A(H5N1) Viruses Isolated from Cats
We experimentally analyzed NAI susceptibility to evaluate the effectiveness of existing influenza antiviral drugs against YS/2023, GA/2023, and YC/2022 viruses. We compared the IC 50 values of the 3 viruses with that of an antiviral drug–susceptible human influenza A(H1N1)pdm09 reference virus. The high sensitivity of YS/2023, GA/2023, and YC/2022 viruses to NAIs confirmed the effectiveness of specific antiviral drugs ( Table 3 ).
HPAI H5N1 outbreaks continue worldwide, posing considerable threats to humans and animals. HPAI H5N1 clade 2.3.4.4b viruses have been detected in wild birds and domestic poultry in South Korea ( 14 ). In addition, infection outbreaks in cats caused by HPAI H5N1 clade 2.3.4.4b viruses occurred in 2 animal shelters in South Korea during July 2023. Both H5N1 viruses isolated from cats had genetic constellations similar to that of the predominant influenza virus circulating in wild birds and poultry in South Korea during 2022–2023. An investigation of the source of infection found that the cats were infected by ingesting raw duck feed contaminated with the prevalent circulating virus. The raw feed–derived viruses were genetically identical to the poultry virus; however, we found 2 mutations related to mammal adaptation (E627K and D701N) in PB2 of the isolates from cats. Therefore, it is critical to prevent HPAI virus infections in mammals because avian-derived influenza viruses have been found to mutate after infecting mammals. We performed genetic analysis and animal model experiments to assess the potential mammal-to-mammal transmission and pathogenicity of HPAI H5N1 clade 2.3.4.4b viruses isolated from cat outbreaks in other mammals.
We analyzed 5 amino acids encoded by the HA gene segment (S123P, S133A, T156A, Q222L, and G224S). S123P, S133A, and T156A have been reported to increase mammal receptor affinity by enhancing binding to α2,6-sialic acid ( 21 ); however, S123P increased the affinity for α2,6-sialic acid only in the presence of E75K, N193K, or R437K substitutions ( 20 ). YC/2022, YS/2023, and GA/2023 viruses did not have E75K, N193K, or R437K substitutions. Clade 2.3.4.4b viruses have not shown increased affinity for α2,6-sialic acid, even with S133A and T156A substitutions in HA (J. Yang et al., unpub. data). We did not find the Q222L and G224S substitutions, which are associated with strong affinity for α2,6-sialic acid, in YC/2022, YS/2023, and GA/2023 viruses ( 20 , 25 ). Consequently, we do not consider the effects of HA mutations to be substantial for those viruses.
The H5N1 PB2 substitution E627K was observed in cats in Poland, and the PB2 D701N substitution was observed in sea lions in Argentina ( 10 , 26 ). According to sequence data registered in GISAID, >50% of human HPAI virus isolates exhibit E627K or D701N substitutions in PB2. Those mammal-adaptive mutations are critical factors that increase replication and virulence of H5N1 viruses in cell culture and animal experiments ( 27 – 30 ).
Ferrets are useful animal models to study influenza virus transmission and are frequently used for influenza pathogenicity evaluation because they exhibit influenza-like symptoms after infection, including fever, malaise, anorexia, sneezing, and nasal discharge ( 31 – 33 ). The H5N1 viruses isolated from cats exhibited high virus replication levels and systemic infection along with severe symptoms and high mortality rates in mice and ferrets; in addition, contact transmission among ferrets was confirmed. Therefore, it was inferred that YS/2023 and GA/2023 are highly pathogenic in mammals and are capable of mammal-to-mammal transmission. It was also presumed that the amino acid substitutions E627K and D701N in PB2, previously associated with increased replication and virulence in mammals ( 29 ), might be responsible for the pathogenicity and transmission of H5N1 viruses in mammals. YS/2023 and the GA/2023 are genetically similar, except for the PB2 substitutions D701N in YS/2023 and E627K in GA/2023. The GA/2023 virus with the E627K substitution showed stronger contact transmission in ferrets than the YS/2023 virus with the 701N substitution. It has been reported that the E627K substitution in PB2 of H5N1 viruses affects airborne transmission in ferrets ( 34 ). Therefore, the E627K mutation might have a greater effect on transmission of the cat-derived viruses than D701N, although this possibility requires further investigation.
We compared the 2 cat-derived viruses with a duck-derived virus that occupied the same clade as the H5N1 virus circulating in poultry during 2022. However, the first limitation of our study is that direct comparisons of pathogenicity between isolates could not be completely assessed because of virus gene segmental differences. Nevertheless, it was clear that the H5N1 viruses isolated from cats were more pathogenic and transmissible among mammals than the duck-derived virus. Second, we analyzed mouse infections and contact transmission in ferrets, but we did not include an aerosol droplet transmission experiment to analyze the potential for human-to-human transmission. Consequently, assessing the public health risk to humans was also limited.
Increasing transmission of H5N1 viruses among mammals has been observed in countries in South America and in the United States. Most cases of spillover into humans have involved direct contact with infected poultry or a contaminated environment. The risk for human infection from recent outbreaks of HPAI influenza viruses in mammals, including tigers, leopards, domestic cats, domestic dogs, sea lions, and seals, has been assessed as low by the World Health Organization and other experts because of the lack of evidence for human-specific adaptive changes ( 6 , 8 , 11 ).
In conclusion, the increased pathogenicity and transmission among mammals observed in ferrets exposed to cat-derived HPAI H5N1 viruses indicate a need to conduct surveillance for H5N1 viruses in wild birds and mammals to prepare for potential zoonotic threats. A One Health surveillance approach is crucial, and sharing and integrating information, such as sequencing data, reference viruses, and experimental data, during outbreaks in birds and mammals are essential to prevent human HPAI H5N1 virus infections.
Dr. Kim is a scientific deputy director of the Division of Emerging Infectious Diseases at the Korea Disease Control and Prevention Agency (KDCA). His main research interests are infectious disease and microbiology.
Acknowledgment
This work was supported by the Korea Disease Control and Prevention Agency (KDCA) (grant no. 6300-6331-301).
- Lee DH , Criado MF , Swayne DE . Pathobiological origins and evolutionary history of highly pathogenic avian influenza viruses. Cold Spring Harb Perspect Med . 2021 ; 11 : a038679 . DOI PubMed Google Scholar
- Caliendo V , Lewis NS , Pohlmann A , Baillie SR , Banyard AC , Beer M , et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci Rep . 2022 ; 12 : 11729 . DOI PubMed Google Scholar
- Lewis NS , Banyard AC , Whittard E , Karibayev T , Al Kafagi T , Chvala I , et al. Emergence and spread of novel H5N8, H5N5 and H5N1 clade 2.3.4.4 highly pathogenic avian influenza in 2020. Emerg Microbes Infect . 2021 ; 10 : 148 – 51 . DOI PubMed Google Scholar
- World Health Organization . Avian influenza weekly update 2024 . Human infection with avian influenza A(H5) viruses [ cited 2024 Mar 8 ]. https://iris.who.int/handle/10665/375483
- Centers for Disease Control and Prevention . Technical report: June 2023 highly pathogenic avian influenza A(H5N1) viruses [ cited 2023 Jul 7 ]. https://www.cdc.gov/bird-flu/php/technical-report/h5n1-070723.html
- Plaza PI , Gamarra-Toledo V , Euguí JR , Lambertucci SA . Recent changes in patterns of mammal infection with highly pathogenic avian influenza A(H5N1) virus worldwide. Emerg Infect Dis . 2024 ; 30 : 444 – 52 . DOI PubMed Google Scholar
- Alkie TN , Lopes S , Hisanaga T , Xu W , Suderman M , Koziuk J , et al. A threat from both sides: Multiple introductions of genetically distinct H5 HPAI viruses into Canada via both East Asia-Australasia/Pacific and Atlantic flyways. Virus Evol . 2022 ; 8 : veac077 . DOI PubMed Google Scholar
- Plaza PI , Gamarra-Toledo V , Rodríguez Euguí J , Rosciano N , Lambertucci SA . Pacific and Atlantic sea lion mortality caused by highly pathogenic Avian Influenza A(H5N1) in South America. Travel Med Infect Dis . 2024 ; 59 : 102712 . DOI PubMed Google Scholar
- Gamarra-Toledo V , Plaza PI , Gutiérrez R , Inga-Diaz G , Saravia-Guevara P , Pereyra-Meza O , et al. Mass mortality of sea lions caused by highly pathogenic avian influenza A(H5N1) virus. Emerg Infect Dis . 2023 ; 29 : 2553 – 6 . DOI PubMed Google Scholar
- Domańska-Blicharz K , Świętoń E , Świątalska A , Monne I , Fusaro A , Tarasiuk K , et al. Outbreak of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in cats, Poland, June to July 2023. Euro Surveill . 2023 ; 28 : 2300366 . DOI PubMed Google Scholar
- Pan American Health Organization . World Health Organization. Risk assessment for public health related to outbreaks caused by highly pathogenic avian influenza (HPAI) A(H5N1), clade 2.3.4.4b, in animal species in the region of the Americas—20 September 2023 [ cited 2023 Sep 20 ]. https://www.paho.org/en/documents/risk-assessment-public-health-related-outbreaks-caused-highly-pathogenic-avian-influenza
- Cha RM , Lee YN , Park MJ , Baek YG , Shin JI , Jung CH , et al. Genetic characterization and pathogenesis of H5N1 high pathogenicity avian influenza virus isolated in South Korea during 2021–2022. Viruses . 2023 ; 15 : 1403 . DOI PubMed Google Scholar
- Lee SH , Cho AY , Kim TH , Ahn SJ , Song JH , Lee H , et al. Novel highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in wild birds, South Korea. Emerg Infect Dis . 2023 ; 29 : 1475 – 8 . DOI PubMed Google Scholar
- Kang YM , Heo GB , An SH , Lee YN , Cha RM , Cho HK , et al. Introduction of multiple novel high pathogenicity avian influenza (H5N1) virus of lade 2.3.4.4b into South Korea in 2022. Transbound Emerg Dis . 2023 ; 8339427 : ••• .
- Lee K , Yeom M , Vu TTH , Do HQ , Na W , Lee M , et al. Characterization of highly pathogenic avian influenza A (H5N1) viruses isolated from cats in South Korea, 2023. Emerg Microbes Infect . 2024 ; 13 : 2290835 . DOI PubMed Google Scholar
- Ministry of Agriculture . Food and Rural Affairs. Feline highly pathogenic avian influenza epidemic prevention area lifted on August 21. Aug 22, 2023 [ cited 2024 Jul 25 ]. https://www.mafra.go.kr/home/5109/subview.do?enc=Zm5jdDF8QEB8JTJGYmJzJTJGaG9tZSUyRjc5MiUyRjU2NzI1OCUyRmFydGNsVmlldy5kbyUzRnJnc0VuZGRlU3RyJTNEJTI2YmJzT3BlbldyZFNlcSUzRCUyNnBhc3N3b3JkJTNEJTI2cGFnZSUzRDEzMSUyNnJnc0JnbmRlU3RyJTNEJTI2cm93JTNEMTAlMjZiYnNDbFNlcSUzRCUyNnNyY2hDb2x1bW4lM0QlMjZpc1ZpZXdNaW5lJTNEZmFsc2UlMjZzcmNoV3JkJTNEJTI2
- Kumar S , Stecher G , Li M , Knyaz C , Tamura K . MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol . 2018 ; 35 : 1547 – 9 . DOI PubMed Google Scholar
- Couzens L , Gao J , Westgeest K , Sandbulte M , Lugovtsev V , Fouchier R , et al. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. J Virol Methods . 2014 ; 210 : 7 – 14 . DOI PubMed Google Scholar
- Govorkova EA , Takashita E , Daniels RS , Fujisaki S , Presser LD , Patel MC , et al. Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2018-2020. Antiviral Res . 2022 ; 200 : 105281 . DOI PubMed Google Scholar
- Yamada S , Suzuki Y , Suzuki T , Le MQ , Nidom CA , Sakai-Tagawa Y , et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature . 2006 ; 444 : 378 – 82 . DOI PubMed Google Scholar
- Suttie A , Deng YM , Greenhill AR , Dussart P , Horwood PF , Karlsson EA . Inventory of molecular markers affecting biological characteristics of avian influenza A viruses. Virus Genes . 2019 ; 55 : 739 – 68 . DOI PubMed Google Scholar
- Gabriel G , Czudai-Matwich V , Klenk HD . Adaptive mutations in the H5N1 polymerase complex. Virus Res . 2013 ; 178 : 53 – 62 . DOI PubMed Google Scholar
- Medina RA , García-Sastre A . Influenza A viruses: new research developments. Nat Rev Microbiol . 2011 ; 9 : 590 – 603 . DOI PubMed Google Scholar
- Holsinger LJ , Nichani D , Pinto LH , Lamb RA . Influenza A virus M2 ion channel protein: a structure-function analysis. J Virol . 1994 ; 68 : 1551 – 63 . DOI PubMed Google Scholar
- Alkie TN , Cox S , Embury-Hyatt C , Stevens B , Pople N , Pybus MJ , et al. Characterization of neurotropic HPAI H5N1 viruses with novel genome constellations and mammalian adaptive mutations in free-living mesocarnivores in Canada. Emerg Microbes Infect . 2023 ; 12 : 2186608 . DOI PubMed Google Scholar
- Rimondi A , Vanstreels RET , Olivera V , Donini A , Lauriente MM , Uhart MM . Highly pathogenic avian influenza A(H5N1) viruses from multispecies outbreak, Argentina, August 2023. Emerg Infect Dis . 2024 ; 30 : 812 – 4 . DOI PubMed Google Scholar
- Subbarao EK , London W , Murphy BR . A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J Virol . 1993 ; 67 : 1761 – 4 . DOI PubMed Google Scholar
- Van Hoeven N , Pappas C , Belser JA , Maines TR , Zeng H , García-Sastre A , et al. Human HA and polymerase subunit PB2 proteins confer transmission of an avian influenza virus through the air. Proc Natl Acad Sci U S A . 2009 ; 106 : 3366 – 71 . DOI PubMed Google Scholar
- Steel J , Lowen AC , Mubareka S , Palese P . Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog . 2009 ; 5 : e1000252 . DOI PubMed Google Scholar
- Pulit-Penaloza JA , Brock N , Pappas C , Sun X , Belser JA , Zeng H , et al. Characterization of highly pathogenic avian influenza H5Nx viruses in the ferret model. Sci Rep . 2020 ; 10 : 12700 . DOI PubMed Google Scholar
- Bodewes R , Kreijtz JHCM , van Amerongen G , Fouchier RAM , Osterhaus ADME , Rimmelzwaan GF , et al. Pathogenesis of Influenza A/H5N1 virus infection in ferrets differs between intranasal and intratracheal routes of inoculation. Am J Pathol . 2011 ; 179 : 30 – 6 . DOI PubMed Google Scholar
- Bodewes R , Rimmelzwaan GF , Osterhaus ADME . Animal models for the preclinical evaluation of candidate influenza vaccines. Expert Rev Vaccines . 2010 ; 9 : 59 – 72 . DOI PubMed Google Scholar
- Thangavel RR , Bouvier NM . Animal models for influenza virus pathogenesis, transmission, and immunology. J Immunol Methods . 2014 ; 410 : 60 – 79 . DOI PubMed Google Scholar
- Herfst S , Schrauwen EJA , Linster M , Chutinimitkul S , de Wit E , Munster VJ , et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science . 2012 ; 336 : 1534 – 41 . DOI PubMed Google Scholar
- Figure 1 . Survival of mice infected with highly pathogenic avian influenza A(H5N1) viruses isolated from cats in South Korea, 2023. Viruses were isolated from 2 cats and 1 duck. A) A/feline/Korea/M302-6/2023;...
- Figure 2 . Virus titers in organs of mice infected with highly pathogenic avian influenza A(H5N1) viruses isolated from cats in South Korea, 2023. Viruses were isolated from 2 cats (YS/2023 and...
- Figure 3 . Survival of ferrets infected with highly pathogenic avian influenza A(H5N1) viruses isolated from cats in South Korea, 2023. Viruses were isolated from 1 duck (YC/2022) and 2 cats (YS/2023...
- Figure 4 . Virus titers in organs of ferrets infected with highly pathogenic avian influenza A(H5N1) viruses isolated from cats in South Korea, 2023. Viruses were isolated from 1 duck (YC/2022) and...
- Figure 5 . Survival rates after ferret-to-ferret contact transmission in study of pathogenicity of highly pathogenic avian influenza A(H5N1) viruses isolated from cats, South Korea, 2023. Viruses were isolated from 2 cats...
- Figure 6 . Virus titers after ferret-to-ferret contact transmission in study of pathogenicity of highly pathogenic avian influenza A(H5N1) viruses isolated from cats, South Korea, 2023. Virus titers were measured in nasal...
- Table 1 . Characteristics of highly pathogenic avian influenza A(H5N1) viruses isolated from cats, South Korea, 2023
- Table 2 . Comparisons of major amino acid substitutions in protein segments from highly pathogenic avian influenza A(H5N1) viruses isolated from cats, South Korea, 2023
- Table 3 . Susceptibility of highly pathogenic avian influenza A(H5N1) viruses isolated from cats in South Korea, 2023, to antiviral drugs
Suggested citation for this article : Kim IH, Nam JH, Kim CK, Choi YJ, Lee H, An BM, et al. Pathogenicity of highly pathogenic avian influenza A(H5N1) viruses isolated from cats in mice and ferrets, South Korea, 2023. Emerg Infect Dis. 2024 Oct [ date cited ]. https://doi.org/10.3201/eid3010.240583
DOI: 10.3201/eid3010.240583
Original Publication Date: September 06, 2024
1 These authors contributed equally to this article.
Table of Contents – Volume 30, Number 10—October 2024
Please use the form below to submit correspondence to the authors or contact them at the following address:
Eun-Jin Kim, Division of Emerging Infectious Diseases, Bureau of Infectious Disease Diagnosis Control, Korea Disease Control and Prevention Agency, 187, Osongsaengmyeong 2-ro, Osong-eup, Heungdeok-gu, Cheongju-si, Chungcheongbuk-do 28159, South Korea
Comment submitted successfully, thank you for your feedback.
There was an unexpected error. Message not sent.
Metric Details
Article views: 130.
Data is collected weekly and does not include downloads and attachments. View data is from .
What is the Altmetric Attention Score?
The Altmetric Attention Score for a research output provides an indicator of the amount of attention that it has received. The score is derived from an automated algorithm, and represents a weighted count of the amount of attention Altmetric picked up for a research output.

IMAGES
COMMENTS
All international travelers should be fully vaccinated against measles with the measles-mumps-rubella (MMR) vaccine, including an early dose for infants 6-11 months, according to CDC's measles vaccination recommendations for international travel. Dogs infected with rabies are not commonly found in Canada.
More. Learn about CDC's Traveler Genomic Surveillance Program that detects new COVID-19 variants entering the country. Sign up to get travel notices, clinical updates, & healthy travel tips. CDC Travelers' Health Branch provides updated travel information, notices, and vaccine requirements to inform international travelers and provide ...
For all travellers entering Canada by air, land or marine mode: Proof of COVID-19 vaccination is not required. Pre-board testing is not required. COVID-19 pre-entry and arrival tests are not required. Quarantine after you enter Canada is not required. Using ArriveCAN is not required, but.
On September 7, 2021, provided that Canada's COVID-19 epidemiology remains favourable, the Government intends to open Canada's borders for discretionary travel by travellers from any country who have been fully vaccinated with Government of Canada-accepted vaccines at least 14 days prior to entering Canada and who meet specific entry ...
On September 7, 2021, provided that Canada's COVID-19 epidemiology remains favourable, the Government of Canada intends to allow discretionary (non-essential) travel by travellers from any country who have been fully vaccinated with Government of Canada-accepted vaccines at least 14 days prior to entering Canada and who meet specific entry ...
November 19, 2021. Today, the Government of Canada announced upcoming adjustments to Canada's border measures. This backgrounder provides additional context to support travellers in understanding COVID-19 testing and vaccine requirements, as well as other border measures, which are an important part of Canada's response to the global COVID ...
All eligible travellers should ideally complete a COVID-19 vaccine series in Canada, along with any additional recommended doses (boosters), at least 14 days before travelling. The COVID-19 vaccines used in Canada are effective at preventing severe illness, hospitalization, and death from COVID-19. They can also provide some protection against ...
Check our Traveler Information Center for more information if you are a traveler with specific health needs, such as travelers who are pregnant, immune compromised, or traveling for a specific purpose like humanitarian aid work. Remember to pack extras of important health supplies in case of travel delays. Prescription medicines. Your prescriptions
The Government of Canada is prioritizing the health and safety of everyone in Canada by taking a risk-based and measured approach to re-opening our borders. On September 7, 2021, provided that the domestic epidemiologic situation remains favourable, the Government intends to open Canada's borders to any fully vaccinated travellers who have completed the full course of vaccination with a ...
Canada has some of the strictest travel and border measures in the world, including a mandatory 14-day quarantine for everyone returning to the country. With new COVID-19 variant detections increasing in the country, the Government of Canada is announcing today further testing and quarantine requirements for international travellers arriving to ...
The Public Health Agency of Canada's Travel Health Notices outline potential health risks to Canadian travellers and recommend ways to help reduce them. Notices remain in effect until removed. Country-specific information on safety and security, local laws and customs, entry requirements, health conditions and other important travel issues.
Objectives. This statement provides guidance for health care providers advising patients on health-related aspects of travel in a COVID-19-affected world. Policy and regulatory aspects of the COVID-19 response, such as requirements around vaccination and quarantine, are outside of the purview of this statement.
Before travelling, talk to a health care provider about your travel plans and how you can reduce your risk of getting viruses like mpox. Travel health notices Up-to-date information on and warnings about rapidly evolving health risks to Canadians travelling or living abroad.
Before you travel, take steps to prepare so you can stay safe and healthy during your trip. Check CDC's destination pages for travel health information. ... Dial 1-888-407-4747 if calling from the United States or Canada, Dial 00 1 202-501-4444 if calling from overseas, or;
The Government of Canada's official source of travel information and advice, the Travel Advice and Advisories help you to make informed decisions and travel safely while you are outside Canada. Check the page for your destination often, because safety and security conditions may change. See Travel Advice and Advisories - FAQ for more ...
If you have questions or need more information, please contact CDC-INFO at (800) 232-4636. The CDC Dog Import Form is the only required form for dogs that have ONLY been in countries that are dog-rabies free or low-risk countries in the 6 months before entering or returning to the U.S. These requirements are the same regardless of vaccination ...
Destinations. Measles cases are increasing globally, including in the United States. The majority of measles cases imported into the United States occur in unvaccinated U.S. residents who become infected during international travel. A list of countries with confirmed measles outbreaks can be found on the Global Measles Travel Health Notice (THN).
Travel outside Canada. Travel documents, travel health and safety, border wait times and more. Air travel. ... What you can bring back to Canada. General guidelines on what you can and cannot bring into Canada when you return from abroad. New rules for dogs entering the U.S. On August 1, 2024, the import requirements for dogs entering the U.S ...
Travelling to Canada. Anyone currently allowed to enter Canada can skip the 14-day quarantine if they meet the country's requirements for being fully vaccinated. That means two doses of either the ...
The U.S. Centers for Disease Control and Prevention (CDC) ... showing a negative COVID-19 test to travel within Canada — rather than proof-of-vaccination — will no longer be an option.
Chang W. Lee/The New York Times. The Centers for Disease Control and Prevention updated its guidance for fully vaccinated Americans in April, saying that traveling both domestically and ...
Advance Declaration: Save time at the border. Use Advance Declaration in ArriveCAN to submit your customs and immigration declaration before flying into Canada. Date modified: 2024-09-06. Government of Canada's official one-stop-shop for comprehensive international travel information.
Here is helpful information on health and safety, travel documents, Canada-U.S border wait times, travelling with children and more. Follow: X; Facebook; Instagram; New rules for dogs entering the U.S. New entry requirements for dogs entering the United States from Canada will take effect on August 1, 2024:
Since the emergence of the highly pathogenic avian influenza (HPAI) A(H5N1) virus (A/chicken/Scotland/59) in Scotland, UK, several outbreaks of H5Nx viruses have been reported in poultry worldwide ().In 1996, an HPAI H5N1 virus, A/goose/Guangdong/1/1996 (Gs/GD), was identified, and the Gs/GD lineage H5 viruses have been circulating in poultry and wild aquatic bird reservoirs for >25 years (1,2).